Claire Patterson as co-lead author in high impact complex targeted nanomedicine (dendrimer) publication
Claire Patterson, Srividya Balachander, Iain Grant et al. present the Design and optimisation of dendrimer-conjugated Bcl-2/xL inhibitor, AZD0466, with improved therapeutic index for cancer therapy The publication describes the use of preclinical and mathematical models to determine the optimal release rate of the drug from the dendrimer, a passively targetted nanomedicine.
The AZD4320-dendrimer conjugate (AZD0466) identified, through mathematical modelling, resulted in an improved therapeutic index and thus enabled progression of this promising dual Bcl-2/Bcl-xL inhibitor into clinical development (NCT04214093).
This work was a collaborative effort between Starpharma (using their DEP® dendrimer platform) and AstraZeneca (where Claire was formerly employed). It exemplifies the potential value add of complex drug delivery systems such as nanomedicine, and in particular, how their design can be guided by pharmacokinetic modelling.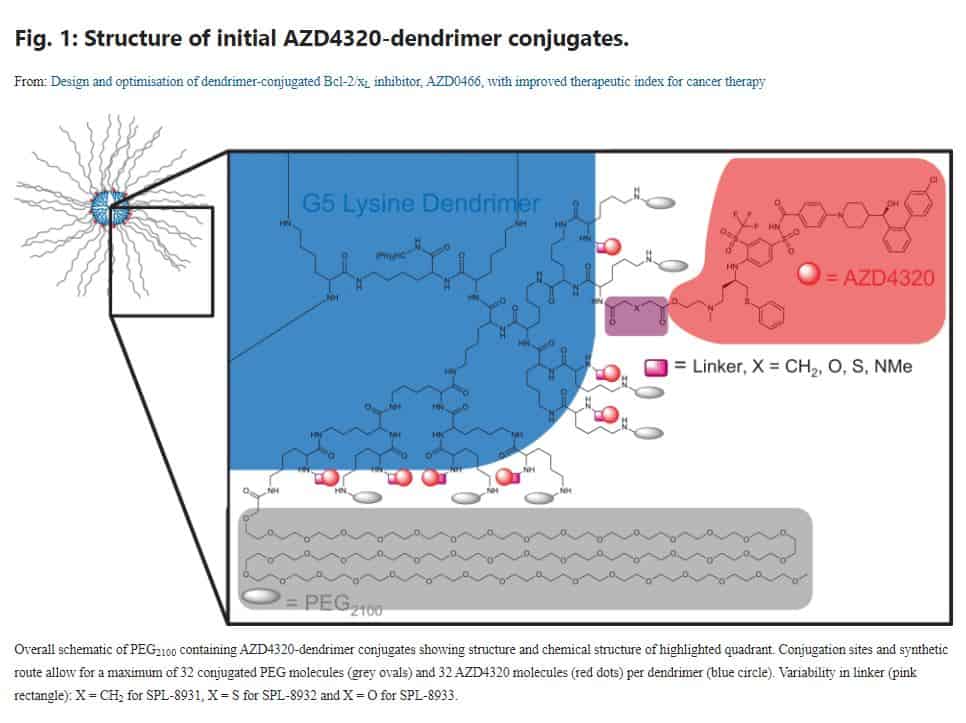
Seda’s team of experts has direct experience of taking several nanomedicine and other complex delivery systems from preclinical to clinical development. We have established a network of experts in the diverse array of advanced characterisation techniques that are so vital to success in this field. In addition, we can leverage in-house modelling and simulation expertise to guide the development and selection of formulation candidates for optimal in vivo performance. Our in-house DMPK and Clinical Pharmacology capability, together with close alliances to other non-clinical and clinical experts are ideal for ensuring the seamless communication required to maximise success of complex medicines, where minor changes in formulation or manufacturing process can have major impact on biodistribution (and hence safety/efficacy).
Find out more about Seda’s complex medicines capabilities.
